2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
4.7 (257) · € 14.99 · In Magazzino
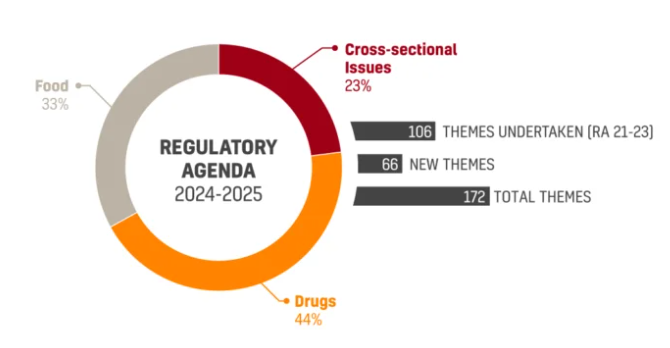
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

ANVISA approves 2024-2025 regulatory agenda - CGM

425

425

News Emergo by UL

Brazil ANVISA Announces Priorities for the 2024-2025 Year

FDA's BsUFA III Features

Human Insulin Drugs Market In Brazil 2023-2028: Market Analysis, Drivers, Restraints, Opportunities, and Threats - 17000 + Technavio Reports
EX-99.1
Yahoo Finance - Stock Market Live, Quotes, Business & Finance News

2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA - Lexology

Vaccines and Related Biological Products Advisory Committee March 5, 2024 Meeting Announcement - 03/05/2024

2021 Q2 Results Presentation & Transcript

Announcements ALTEX - Alternatives to animal experimentation







